Product safety and CE conformity
The General Product Safety Directive 2001/95/EC applies to all products for which there is no more specific legislation. This Directive defines general requirements for the safety of the product for use. Under this Directive, EU manufacturers and importers are obliged to ensure that only safe, sound and high quality products are placed on the market.
The CE conformity system is an integral part of the European product safety legislation. The implementation of the CE process is always the responsibility of the manufacturer or importer. In addition, the CE marking improves trade in the common market of the European Union, as all member states are obliged to prevent the implementation of additional laws relating to CE-relevant products.

CE conformity assessment
The CE marking system knows more than a conformity assessment procedure. The procedures which the responsible body may follow are those laid down in the applicable regulations and directives.
Extract of important CE regulations:
1. 2009/48/EC Toy Directive
2. 2014/35/EU Low-voltage devices (electronics with external power supply)
3. 2014/30/EU Electromagnetic compatibility (electronics)
4. 2014/53/EU Radio Directive (e.g. electronics with Bluetooth)
5. 2011/65/EU RoHS Directive (Electronics)
6. 2009/125/EC Ecodesign Directive (e.g. external power supplies)
7th Regulation (EU) 2016/425 Personal protective equipment (e.g. spectacles)
Each Directive defines in the Annex the applicable conformity assessment procedures referred to as “modules”. In addition, all modules are also explained in Decision No 768/2008/EC.
As a rule, the manufacturers themselves can decide which module to use. The selection should take into account criteria such as the degree of complexity of the product, the number of batches and the production volume, the internal know-how and the equipment for carrying out the conformity process.
Decision No 768/2008 contains a total of 16 modules. Modules A, A1, A2 (Internal Production Control) can be used for most products. The difference between A, A1 and A2 is the extent of testing and process evaluation, which depends on the production method used (e.g. fully automated production) and the product specification. (e.g. safety critical measurements or material).
In general, the CE conformity assessment shall be carried out according to the following steps:
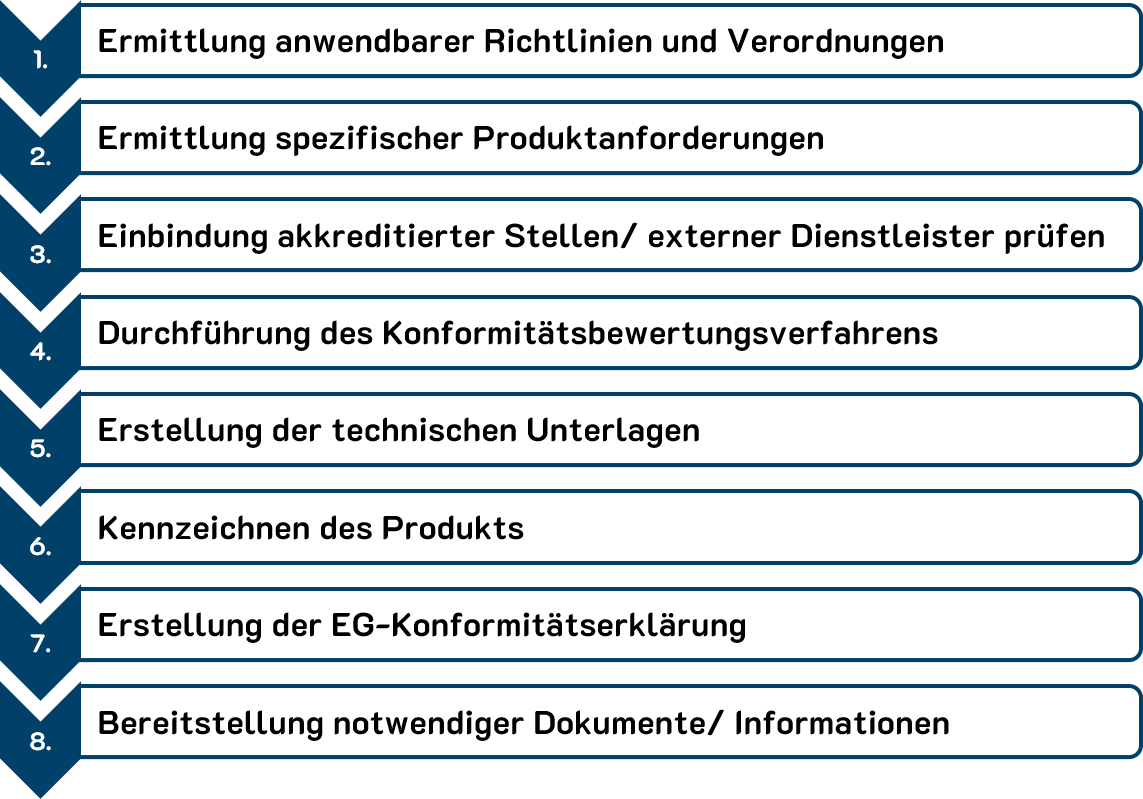
CE declaration of conformity
There is no official form of the EC Declaration of Conformity, but the Annexes to the European CE Directives define the minimum requirements for the content. In case of applicability of more than one CE Directive, the manufacturer chooses the annex with the strictest declaration requirements.
The name of the signatory should be clearly legible. The person should be an authorised signatory, a quality control manager or even a managing director.
Technical documentation
As a rule, the CE conformity assessment begins with product development. In this phase it is no problem to collect relevant technical drawings and to prepare the risk analysis or other relevant documents.
An overview of the required technical documentation can be found in the annex to the applicable CE regulation or directive.
Practical recommendations
Producers and manufacturers should ensure that a risk analysis is made available at an early stage – this test can save a lot of work in the CE conformity procedure in the long term.
In addition, manufacturers and producers should check the validity of references (standards, directives) of their existing CE declarations at least once a year.
In order to maintain CE conformity and for change management, it is particularly important to note that any product changes made by the manufacturer after production releases have been issued and product tests carried out should already be taken into account during the planning phase. Otherwise, a reassessment and first-of-production approval is required. This also includes any change of (sub-)supplier and/or material which may require new initial samples, product tests and documentation.

